Established in 2014, we are committed to improving the diagnostic experience of patients and healthcare professionals around the globe with innovative Digital Health ready solutions that enable precise diagnosis, early detection, and prevention.
Tomorrow is now — embodied with medical grade wearables, big data, AI-enabled algorithm, our 14-day continuous patch ECG technology, and Professional-reviewed ECG reports are supporting Cardiologists and Neurologists in Asia, and shifting the Arrhythmia Diagnosis paradigm in the Region.

SIGKNOW
Diagnostic Solution Provider
Diagnostic Solution Provider
OUR SERVICE
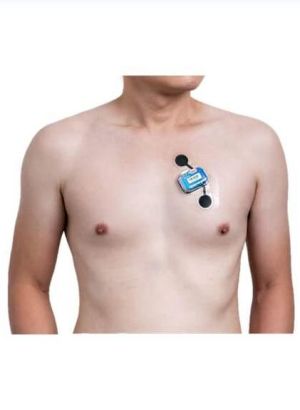
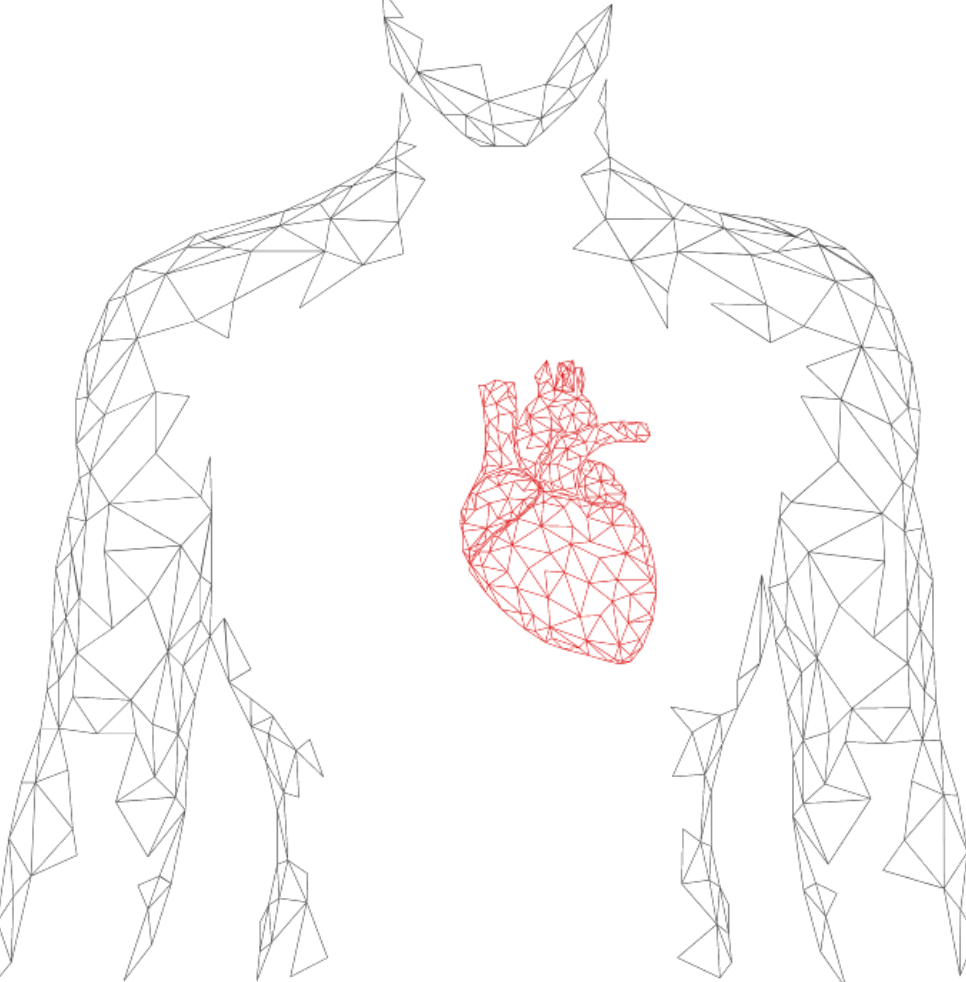
Unlock our primary solution for your heart – EZYPRO® Continuous ECG Monitor,
and colorectal condition screening at home – JustWipe® FOBT Kit.
We work and care for health professionals' workflow, and strive to satisfy the clinical need, enhance the testing convenience, optimize current modalities, and build up a harmonious doctor-patient relationship.
and colorectal condition screening at home – JustWipe® FOBT Kit.
We work and care for health professionals' workflow, and strive to satisfy the clinical need, enhance the testing convenience, optimize current modalities, and build up a harmonious doctor-patient relationship.
Certified ECG Analysis Center
A sophisticated fusion of deep learning software and certified ECG analysts. Our quality and analysis competence follow international guidelines. EZYPRO® Reporting is praised by global cardiologists.

14-Day Continuous ECG Recording
Extend the traditional 24-hour recording to 14-day continuous monitoring. The solution demonstrates high patient compliance and arrhythmia detection. EZYPRO® looks after your heart.
QUALITY AND CERTIFICATION













EZYPRO® Continuous ECG Patch Monitor can customize your monitoring time (1/3/7/14 days) without disturbing your daily activities. Complete with professional ECG reporting, the arrhythmia diagnostics for cardiologists.
In-home fecal occult blood test kit. World 1st toilet-paper-like test kit to collect your feces sample. Improve patients' collection experience and raise consciousness for early colorectal conditions. The favorable screening tool you can't live without.
IMPORTANT MILESTONES
-
2014Sigknow Biomedical Co., Ltd. founded in June Linkou, New Taipei City

-
2016Setup HQ in Songshan TaipeiEZYPRO® UG01 Pilot Production
-
2017EZYPRO® UG01 TFDA & CE approvedISO 13485 certifiedEZYPRO® Software GMP certified

-
2018Post Market Clinical Trials with Leading Medical CentersEZYPRO® UG01 : 2018 National Innovation Award

-
2019UG01 Commercialization in TaiwanEZYPRO® UG02 TFDA approvedEZYPRO® UG01 : 2019 National Innovation Award-the Excelsior Award

-
2020Malaysia MDA listed : 1st Oversea Market followed by ASEAN expansionEZYPRO® UG02 CE approvedEZYPRO® UG01 & JustWipe : 2021 Taiwan Excellence

-
2021Czech SUKL listed : Start European Market PilotISO 27001 certifiedEZYPRO® UG02 : 2022 Taiwan Excellence

-
2022FDA approved in June

14
Dec.2017
Company News
準訊生醫潛血試紙獲2017國家新創獎肯定
準訊生醫結合長庚大學醫工所產學團隊研發出一款以衛生紙為載體的糞便潛血檢測試紙-擦即測,衛生紙式的設計將糞便潛血檢測融入每日如廁的行為當中。